LNNano is committed to the development of nanomaterials and new functional materials on a safe and sustainable basis in the direction of responsible innovation and Safe-by-Design. In this sense, we offer facilities and technical-scientific support to study the effects of nanomaterials on biological systems and the environment; aiming at a proactive assessment of potential hazards (toxicity) and risks involved during the development of these materials, including their functionalized derivatives, by-products and waste (life cycle). We support the generation of innovative products in Brazil in harmony with the protection of human, animal and environmental health.
The Nanotoxicology and Nanosafety Facility develops and employs alternative methods to the use of animals in scientific experimentation – Principle 3Rs (Replacement, Reduction and Refinement). This facility has biosafety level NB-2 and provides personal protective equipment (PPE) for all its users; in addition to maintaining high standards of good laboratory practices (GLP) to guarantee the quality of research projects, results and services performed.
Infrastructure
Dynamic and Electrophoretic Light Scattering (Zetasizer Ultra)
Description
Fundamental equipment for characterization of nanoparticles, nanomaterials, polymers and biomolecules dispersed in a liquid medium such as water, organic solvents, buffers and biological media in general. The study of the toxicity of any type of nanostructure requires an understanding of its interaction with the culture medium and biological fluids (characterization of size, electrical charge, biocoronas formation and aggregation/agglomeration phenomena). Furthermore, for the execution of many protocols and standardized tests (toxicity, safety and regulation) it requires the characterization of nanomaterial dispersions using Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS) techniques. The Zetasizer Ultra equipment has a new technology to measure particle size in scatterings through fast multi-angle dynamic light scattering (MADLS®) measurements, which significantly increases the precision and accuracy of size measurements. In addition, Zetasizer Ultra features a method for determining the concentration/number of particles in dispersion.
Specifications
Multi-Angle Dynamic Light Scattering (MADLS) and Electrophoretic Light Scattering (ELS) – (Zetasizer Ultra, Malvern, UK).
– Angles: 173°, 13° and 90°.
– Diameters achieved: 0.3 nm to 15 μm.
– Minimum concentration: 0.1 mg/ml or 15 kDa (protein).
– Measurements of the zeta potential of particles between 3.8 nm to 100 μm.
– Particle concentration analysis: from 1×108 to 1×1012 particles/mL.
– Accessories: automatic titrator (MTP-3) and viscometer (SV-10).A703
Description
Specifications
Dynamic and Electrophoretic Light Scattering (Zetasizer Ultra)
Description
Fundamental equipment for characterization of nanoparticles, nanomaterials, polymers and biomolecules dispersed in a liquid medium such as water, organic solvents, buffers and biological media in general. The study of the toxicity of any type of nanostructure requires an understanding of its interaction with the culture medium and biological fluids (characterization of size, electrical charge, biocoronas formation and aggregation/agglomeration phenomena). Furthermore, for the execution of many protocols and standardized tests (toxicity, safety and regulation) it requires the characterization of nanomaterial dispersions using Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS) techniques. The Zetasizer Ultra equipment has a new technology to measure particle size in scatterings through fast multi-angle dynamic light scattering (MADLS®) measurements, which significantly increases the precision and accuracy of size measurements. In addition, Zetasizer Ultra features a method for determining the concentration/number of particles in dispersion.
Specifications
Multi-Angle Dynamic Light Scattering (MADLS) and Electrophoretic Light Scattering (ELS) – (Zetasizer Ultra, Malvern, UK).
– Angles: 173°, 13° and 90°.
– Diameters achieved: 0.3 nm to 15 μm.
– Minimum concentration: 0.1 mg/ml or 15 kDa (protein).
– Measurements of the zeta potential of particles between 3.8 nm to 100 μm.
– Particle concentration analysis: from 1×108 to 1×1012 particles/mL.
– Accessories: automatic titrator (MTP-3) and viscometer (SV-10).A703
Description
Specifications
Sedimentation by Differential Centrifugation (CPS Disc Centrifuge)
Description
The Differential Centrifugal Sedimentation (DCS) technique is capable of analyzing the size of particles in aqueous suspensions with high resolution. Through high-speed disk centrifugal force (up to 24,000 rpm), particles in the range of 3 nm to 50 µm can be accurately characterized. This technique is an excellent tool to monitor the size of nanoparticles and agglomeration/aggregation processes of materials in biological media and fluids, such as mineral water, cell culture media, human plasma and among other media of interest. In the field of nanotoxicology and nanobiotechnology, for example, the DCS technique can be used to characterize the interaction of proteins with the surface of nanoparticles (biocoronas formation) and monitor the colloidal stability of dispersions (agglomeration phenomena).
Specifications
•Differential Centrifugal Sedimentation (DCS) – CPS
Disc Centrifuge, Mod. DC24000UHR (CPS Instruments Inc., Prairieville, USA).
• Maximum and minimum disk speed: 24000 rpm
and 600 rpm, respectively.
• Maximum and minimum measurable size: > 40 μm and
<0.01 µm, respectively.
• Minimum and typical resolution (size difference
for narrow peaks): 10% and <5%, respectively.
• Minimum and typical accuracy and repeatability (comparable to calibration standards): ± 2% and ± 0.5%, respectively.
Description
Specifications
Sedimentation by Differential Centrifugation (CPS Disc Centrifuge)
Description
The Differential Centrifugal Sedimentation (DCS) technique is capable of analyzing the size of particles in aqueous suspensions with high resolution. Through high-speed disk centrifugal force (up to 24,000 rpm), particles in the range of 3 nm to 50 µm can be accurately characterized. This technique is an excellent tool to monitor the size of nanoparticles and agglomeration/aggregation processes of materials in biological media and fluids, such as mineral water, cell culture media, human plasma and among other media of interest. In the field of nanotoxicology and nanobiotechnology, for example, the DCS technique can be used to characterize the interaction of proteins with the surface of nanoparticles (biocoronas formation) and monitor the colloidal stability of dispersions (agglomeration phenomena).
Specifications
•Differential Centrifugal Sedimentation (DCS) – CPS
Disc Centrifuge, Mod. DC24000UHR (CPS Instruments Inc., Prairieville, USA).
• Maximum and minimum disk speed: 24000 rpm
and 600 rpm, respectively.
• Maximum and minimum measurable size: > 40 μm and
<0.01 µm, respectively.
• Minimum and typical resolution (size difference
for narrow peaks): 10% and <5%, respectively.
• Minimum and typical accuracy and repeatability (comparable to calibration standards): ± 2% and ± 0.5%, respectively.
Description
Specifications
Darkfield Hyperspectral Microscopy (CytoViva)
Description
Enhanced Darkfield Hyperspectral Microscopy (EDHM) is a system for the identification of nanoparticles after interaction with biological systems and diverse complex matrices. This equipment combines darkfield optical microscopy (VNIR 400-1000 nm) and fluorescence techniques for high-resolution spectral imaging (CytoViva® System). Through advanced analysis software, a spectral library with a nanometric resolution of the material under study is generated from its light scattering spectra. Once the spectral signature of the nanomaterial of interest is obtained, it is possible to identify it in different matrices, such as cells and biological tissues. This identification is based on the spectral identity of the nanomaterial (library), not having the need to use contrasts or fluorophores to mark the nanomaterial under study. In this way, it is possible to differentiate isolated nanoparticles from functionalized nanoparticles coated with biomolecules or in aggregates, once their spectral identity is altered. However, patterns and morphological changes in biological tissues can also be monitored using EDHM – CytoViva microscopy (CytoViva Inc., Auburn, USA);
• Dark-field optical microscope (Olympus, Model BX53) equipped with 10, 40, 63 and 100x objectives and fluorescence filters at the wavelengths of the fluorophores DAPI (blue), Texas Red (red) and FITC (green);
• Spectral camera ranging from 400 to 1000 nm (VNIR) and spectral resolution of 1.5 nm (with 30 μm aperture). Maximum spatial scan width of 896 µm at 10x magnification;
• Pixel size: 6.45 μm x 6.45 μm;
• Resolution: 1392 x 1040;
• Exposure time: from 5 μs to 60 sec;
• Image analysis software: Envi 4.8.
Specifications
• Enhanced dark-field hyperspectral microscopy (EDHM) – CytoViva microscopy (CytoViva Inc., Auburn, USA);
• Dark-field optical microscope (Olympus, Model BX53) equipped with 10, 40, 63 and 100x objectives and fluorescence filters at the wavelengths of the fluorophores DAPI (blue), Texas Red (red) and FITC (green);
• Spectral camera ranging from 400 to 1000 nm (VNIR) and spectral resolution of 1.5 nm (with 30 μm aperture). Maximum spatial scan width of 896 µm at 10x magnification;
• Pixel size: 6.45 μm x 6.45 μm;
• Resolution: 1392 x 1040;
• Exposure time: from 5 μs to 60 sec;
• Image analysis software: Envi 4.8.
Description
Specifications
Darkfield Hyperspectral Microscopy (CytoViva)
Description
Enhanced Darkfield Hyperspectral Microscopy (EDHM) is a system for the identification of nanoparticles after interaction with biological systems and diverse complex matrices. This equipment combines darkfield optical microscopy (VNIR 400-1000 nm) and fluorescence techniques for high-resolution spectral imaging (CytoViva® System). Through advanced analysis software, a spectral library with a nanometric resolution of the material under study is generated from its light scattering spectra. Once the spectral signature of the nanomaterial of interest is obtained, it is possible to identify it in different matrices, such as cells and biological tissues. This identification is based on the spectral identity of the nanomaterial (library), not having the need to use contrasts or fluorophores to mark the nanomaterial under study. In this way, it is possible to differentiate isolated nanoparticles from functionalized nanoparticles coated with biomolecules or in aggregates, once their spectral identity is altered. However, patterns and morphological changes in biological tissues can also be monitored using EDHM – CytoViva microscopy (CytoViva Inc., Auburn, USA);
• Dark-field optical microscope (Olympus, Model BX53) equipped with 10, 40, 63 and 100x objectives and fluorescence filters at the wavelengths of the fluorophores DAPI (blue), Texas Red (red) and FITC (green);
• Spectral camera ranging from 400 to 1000 nm (VNIR) and spectral resolution of 1.5 nm (with 30 μm aperture). Maximum spatial scan width of 896 µm at 10x magnification;
• Pixel size: 6.45 μm x 6.45 μm;
• Resolution: 1392 x 1040;
• Exposure time: from 5 μs to 60 sec;
• Image analysis software: Envi 4.8.
Specifications
• Enhanced dark-field hyperspectral microscopy (EDHM) – CytoViva microscopy (CytoViva Inc., Auburn, USA);
• Dark-field optical microscope (Olympus, Model BX53) equipped with 10, 40, 63 and 100x objectives and fluorescence filters at the wavelengths of the fluorophores DAPI (blue), Texas Red (red) and FITC (green);
• Spectral camera ranging from 400 to 1000 nm (VNIR) and spectral resolution of 1.5 nm (with 30 μm aperture). Maximum spatial scan width of 896 µm at 10x magnification;
• Pixel size: 6.45 μm x 6.45 μm;
• Resolution: 1392 x 1040;
• Exposure time: from 5 μs to 60 sec;
• Image analysis software: Envi 4.8.
Description
Specifications
Toxicity – Zebrafish (ZFET)
Description
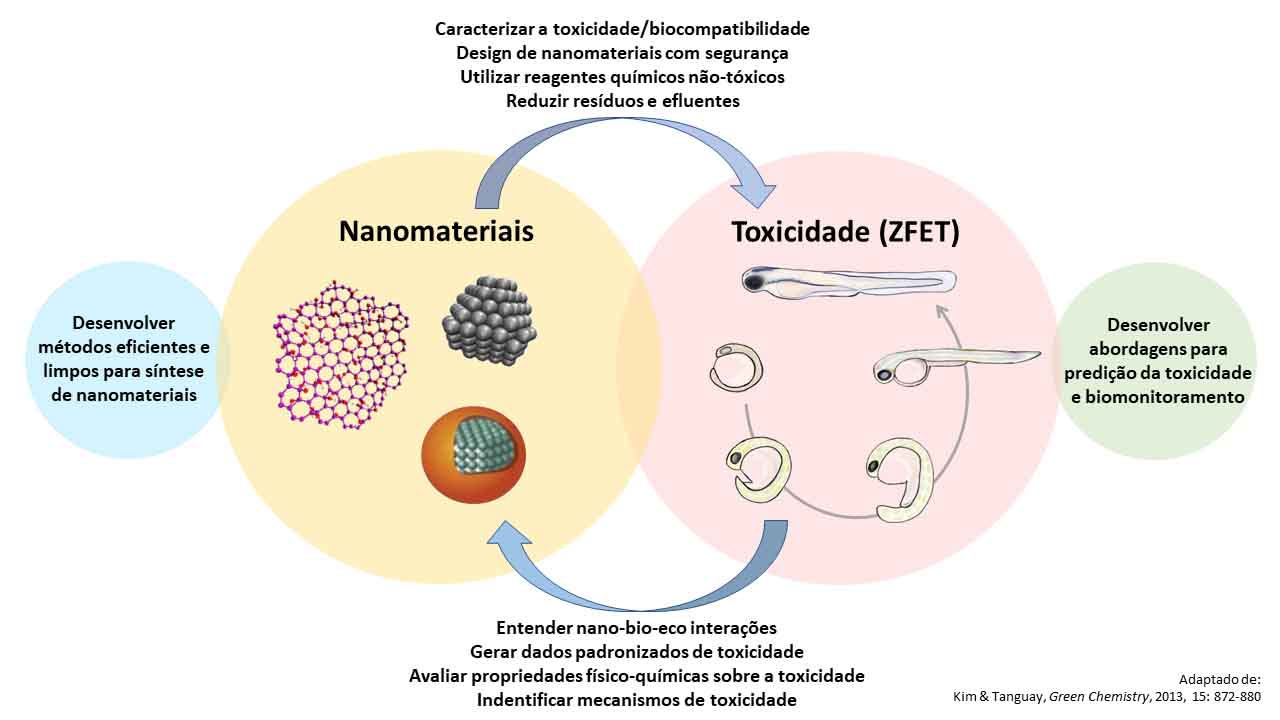
Nanomaterials have diversified technological applications and great potential for innovation in multiple industrial sectors: health, energy, advanced materials, electronics, cosmetics, agriculture, environment, among others. However, the assessment of (nano)toxicity is essential to support risk management procedures, safety and regulations, thus ensuring the commercial exploitation of these materials on a safe and sustainable basis.
Zebrafish is an important biological model for characterizing the toxicity of chemical compounds and nanomaterials in general. In this sense, LNNano has implemented a protocol for carrying out toxicity tests using Zebrafish embryos (ZFET) and which is available to external users. The use of the ZFET assay is a promising strategy to advance the safe-by-design concept, and in particular, the application of green chemistry principles during the development of nanotechnology and new materials.
Specifications
• Zebrafish Embryo Acute Toxicity Test (ZFET): excellent method for evaluating the toxicity and/or biocompatibility of nanomaterials, commonly used in toxicology with a biological model (both for biomedical and environmental areas);
• Species: Danio rerio, popularly known as Zebrafish;
• Freshwater fish with 70% genetic homology with humans;
• Its high reproductive rate and similarity to humans make it an alternative model to the use of rodent animals;
• Meets the criteria of the 3Rs (Replacement, Refinement and Reduction);
• Parameters monitored in the ZFET: mortality, hatching rate, growth, deformation and presence of edema;
•Exposure time: 96 h; Temperature: 28 °C and Photoperiod: 14/10 h (light/dark).
Description
Specifications
Toxicity – Zebrafish (ZFET)
Description
Nanomaterials have diversified technological applications and great potential for innovation in multiple industrial sectors: health, energy, advanced materials, electronics, cosmetics, agriculture, environment, among others. However, the assessment of (nano)toxicity is essential to support risk management procedures, safety and regulations, thus ensuring the commercial exploitation of these materials on a safe and sustainable basis.
Zebrafish is an important biological model for characterizing the toxicity of chemical compounds and nanomaterials in general. In this sense, LNNano has implemented a protocol for carrying out toxicity tests using Zebrafish embryos (ZFET) and which is available to external users. The use of the ZFET assay is a promising strategy to advance the safe-by-design concept, and in particular, the application of green chemistry principles during the development of nanotechnology and new materials.
Specifications
• Zebrafish Embryo Acute Toxicity Test (ZFET): excellent method for evaluating the toxicity and/or biocompatibility of nanomaterials, commonly used in toxicology with a biological model (both for biomedical and environmental areas);
• Species: Danio rerio, popularly known as Zebrafish;
• Freshwater fish with 70% genetic homology with humans;
• Its high reproductive rate and similarity to humans make it an alternative model to the use of rodent animals;
• Meets the criteria of the 3Rs (Replacement, Refinement and Reduction);
• Parameters monitored in the ZFET: mortality, hatching rate, growth, deformation and presence of edema;
•Exposure time: 96 h; Temperature: 28 °C and Photoperiod: 14/10 h (light/dark).
Description
Specifications